New report: 25 major U.S. medical universities violate key transparency law
Twenty-five leading U.S. universities are violating a key medical research transparency law, a new report released today by TranspariMED and Universities Allied for Essential Medicines shows.
Between them, these universities have failed to post the results of 140 out of 450 clinical trials subject to legal reporting requirements.
Hidden and incompletely reported clinical trial results harm patients and undermine public health, but only 15 out of forty universities assessed have posted the results of all of their due trials onto a public database as required by law, the new analysis shows.
The other 25 universities are in breach of the Food and Drug Administration Amendments Act, whose Final Rule came into force in early 2017. For example, Columbia University has not posted the results of 83% of its trials.
In terms of the largest number of trials left unreported, the University of California San Francisco has not posted the results of 17 trials onto the public database, Clinicaltrials.gov. Other major violators include Columbia University, Mayo Clinic, New York University, and the MD Anderson Cancer Center at the University of Texas.

The list of universities that fully comply with the legal, ethical and scientific requirement to report their trial results includes the University of North Carolina Chapel Hill, Emory University, and Johns Hopkins.
Dr Tedros Adhanom Ghebreyesus, WHO’s Director-General, said:
“We advocate full transparency of which clinical trials are ongoing and ensuring all results are disclosed in a timely manner in accordance with the WHO Joint Statement on disclosure of results from clinical trials. This is consistent with the principal goal of medical research: to serve the betterment of humanity. In the case of clinical trials, full transparency on results advances both scientific understanding and timelines for product development and ultimately enables access to essential medicines. We would welcome Universities joining as signatories.”
Till Bruckner, founder of TranspariMED, said:
“Patients and taxpayers are paying a steep price while law-breaking companies and universities walk free. The FDA should start by enforcing the law by imposing fines immediately to prevent important medical discoveries from becoming lost forever.”
Merith Basey, Executive Director of UAEM North America, said:
“As institutions with social missions, universities are morally bound to be transparent with their research outcomes especially when trials are publicly-funded in the first place. We are urging universities to step up their commitments by becoming the first universities worldwide to sign onto the WHO Joint Statement, following in the footsteps of leading groups like Doctors without Borders, Drugs for Neglected Diseases Initiative and The Wellcome Trust.”
University reactions, as reported by STAT News:
UCSF - Investigators leading unreported trials have been notified, compliance will “steadily improve"
MD Anderson - "[W]e will continue efforts to complete data publication for our remaining trials within the shortest time possible"
Mayo Clinic - "We are currently reviewing the cases noted in the report and will do whatever is necessary to close the loop.”
Click here for the full report and methodology, including an overview of the legal framework and an explanation of why posting clinical trial results onto registries in particular is so important. The press release can be found here.
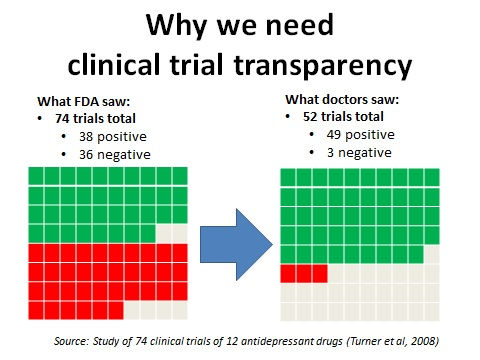
To learn more about why clinical trial transparency is crucial to patients' wellbeing, public health and medical progress, click on the slideshow below.